
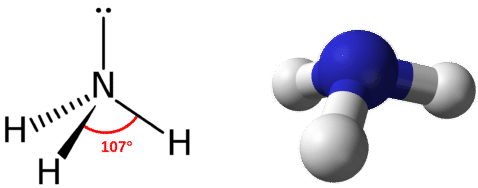
If we compare electronegativities, Hydrogen has less value than Nitrogen. The total number of valence electrons in an NH2- anion = 5 + 2*1 + 1 = 8. Other than this, we have an electron that gives us the negative charge of -1.

Nitrogen belongs to period 15 and has 5 valence electrons whereas hydrogen belongs to period 1 and has only 1 valence electron. Let us start drawing lewis structure for NH2 While finding out the pictorial representation of the molecule, it is a crucial step to check whether the atoms are in their least possible formal charge values. If we assume that electrons will always be shared in equal proportions between atoms while forming a molecular structure, then the individual electric charge assigned to the atoms is said to be the formal charge. However, there are certain exceptions to this rule. This is known as the octet rule or octet fulfillment. they tend to achieve a valency of 8 while bond formation. These elements have a tendency to attain the outer shell electronic configuration of noble gas elements, i.e. If we have a look into the modern periodic table, we can see that the main group elements are present between groups 1-17. We denote these as dots while drawing the Lewis Structure, also known as electron-dot structure. Valence electrons refer to the electrons in the outermost shell of an atomic nucleus. Accordingly, there can be single, double, and triple bonds. When two atoms share electron pairs, a bond is formed. It is defined to be the combining capacity of an atom to form bond structures. Valence electrons and electron dot notations: Here, we have three key concepts to understand before we can proceed to sketch the Lewis Structure of any given molecule, in this case, azanide ion.ġ. Lewis Structure is a 2D diagram to represent the internal bonding between constituent atoms in a molecule or ion. The most initial and important step towards finding out the bonding nature of any chemical molecular structure is to draw its Lewis Structure. Let us now get into our topic of discussion and talk about the chemical bonding nature of NH2. ( Amidyl group is formed due to a loss of a proton from NH2-). NH2- is not stable as such and therefore it is found to exist as a hydrazine compound.Īmidyl group( -NH-) and azanidylidene group (=N-) are substituent groups from azanide. The above reaction shows how metal amides like Lithium Amide are being produced from liquid ammonia solution and Li metal.Īzanide is a monovalent and inorganic anion having a negative charge of -1. NH2- is the conjugate acid of hydridonitrate (H-N2-) and conjugate base of ammonia (NH3). Here, in this article, we will focus on the azanide anion (NH2-). It has a molar mass of around 16.0226 g/mol and is the neutral form of amide ions or azanide ions. This property makes this radical short-lived (unless treated with certain organic molecules).

As a neutral compound, this is known to exist as a radical known as amino radical and therefore has the formula NH2.Ī radical is known to consist of an unpaired valence electron and therefore tends to be highly chemically reactive. However, this has several forms of existence as chemical entities. NH2, as we can see, is a chemical composition of nitrogen and hydrogen atoms.


 0 kommentar(er)
0 kommentar(er)
